Products
PRODUCTS
BIOLOGICS
-
Biosimilar
EPO
-
Recombinant
Factor Ⅷ
-
Biosimilar
Aflibercept
-
Recombinant
Anti-SFTSV Ab
-
Biosimilar
G-CSF
Panpotin® Epoetin alfa (True Epoetin Alfa Biosimilar)
-
-
- Panpotin® was developed under the "totality of the evidence" criteria, proving equivalence to Eprex®/Erypo® in quality, safety, and efficacy, per the EMA and MFDS EPO biosimilar guideline.
- Panpotin® is the only Korea Epoetin alfa biosimilar posted as a biosimilar by the MFDS on the International Pharmaceutical Regulatory Forum (IPRP) site.
- Panpotin®, an approved biosimilar epoetin alfa, was authorized in Korea (2019, 2023), Malaysia (2019), Philippines (2022), Saudi Arabia (2023), Myanmar (2023), Brunei (2024), Thailand (2024), and Türkiye (2024).
Similar PK and PD profiles of Panpotin® compared to Eprex® in Phase 1 clinical study
- Results from Phase 1 demonstrated that Panpotin® has similar pharmacokinetics and pharmacodynamic profiles as reference product Eprex® in healthy volunteers (n=27).
Phase1
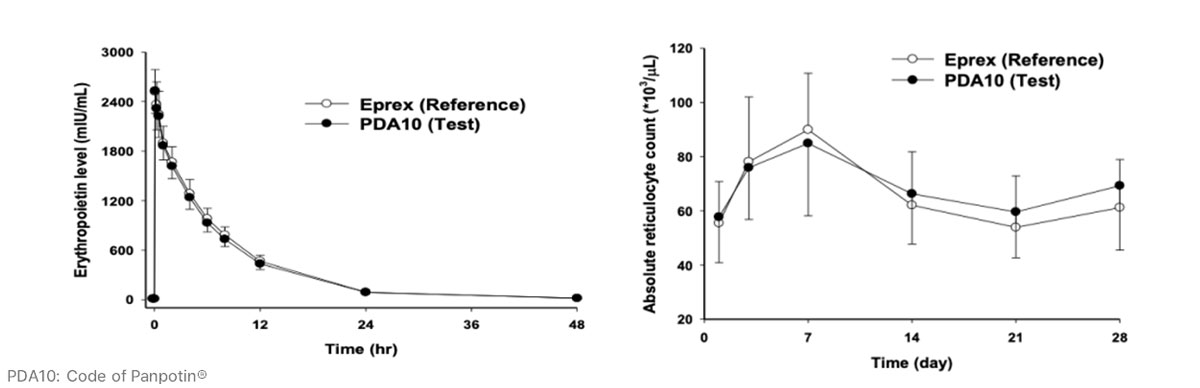
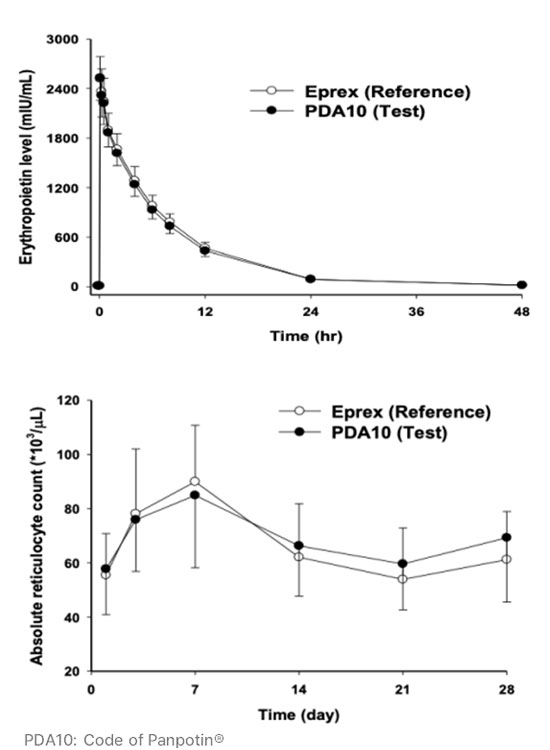
* Reference: Oh, M., Yoon, J., & Cho, D. Y. (2015). Pharmacokinetic and pharmacodynamic comparison of two recombinant human erythropoietin formulations, PDA10 and Eprex, in healthy Korean male volunteers: A randomized, double-blinded, single-dose, two-period crossover study. Clinical Drug Investigation, 35(10), 659–664.
Similar efficacy and safety attributes of Panpotin® compared to Eprex® in phase 3 clinical study
- Results from Phase 3 demonstrated that Panpotin® has similar clinical efficacy and safety as reference product Eprex® in ESRD patients (n=298) on hemodialysis during 28 weeks, with hemoglobin levels and weekly dosages stably maintained after switching to Panpotin® during a 24-week open-label extension period.
Phase3
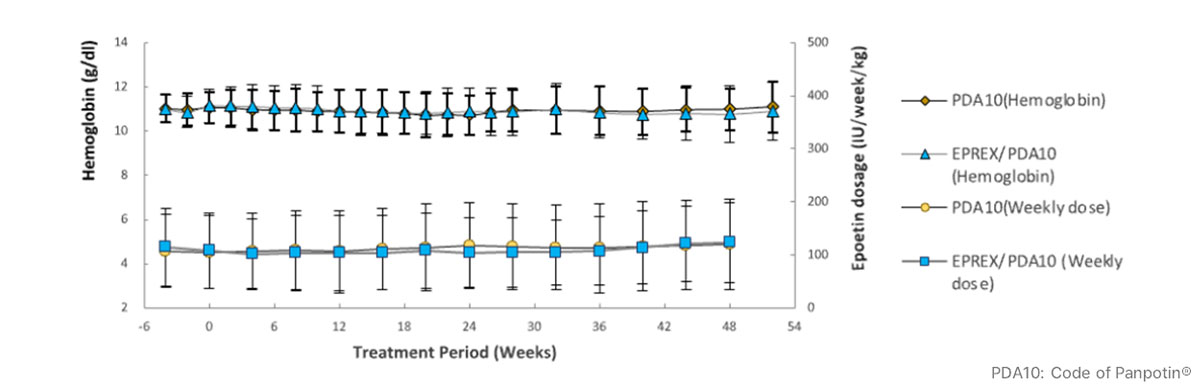
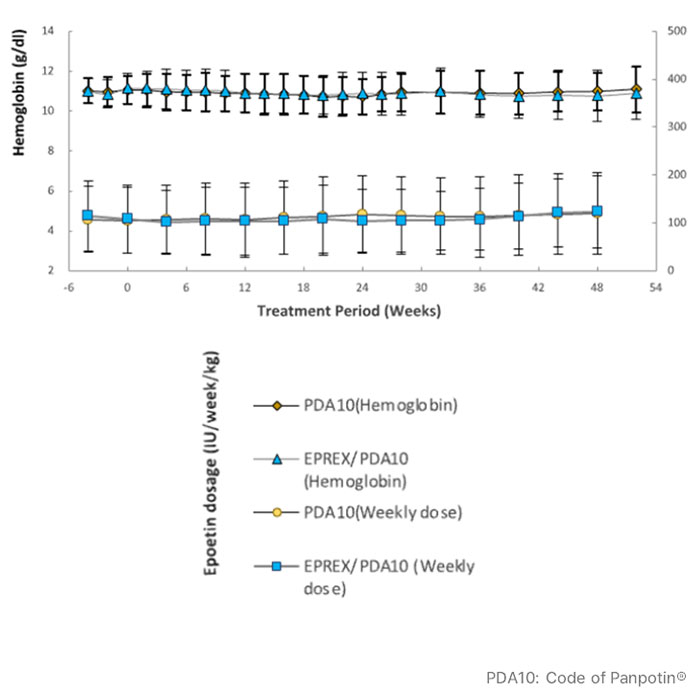
* Reference: im, S. K., Goh, B. L., Visvanathan, R., Kim, S. H., Jeon, J. S., Kim, S. G., Chang, J. H., Lim, C. S., & Morad, Z. (2021). A multicentre, multi-national, double-blind, randomised, active-controlled, parallel-group clinical study to assess the safety and efficacy of PDA10 (Epoetin-Alfa) vs. Eprex® in patients with anaemia of chronic renal failure. BMC Nephrology, 22, 391.



