R&D
R&D
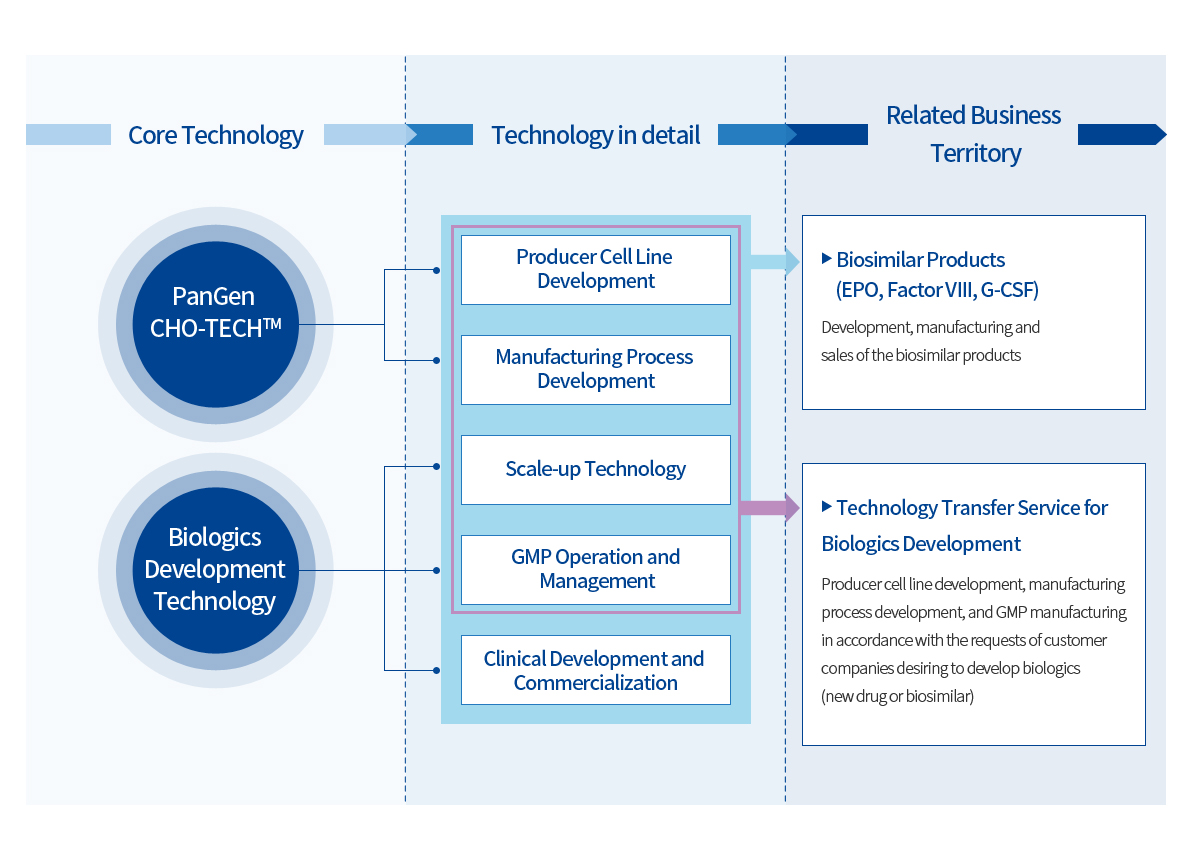
Core Technology
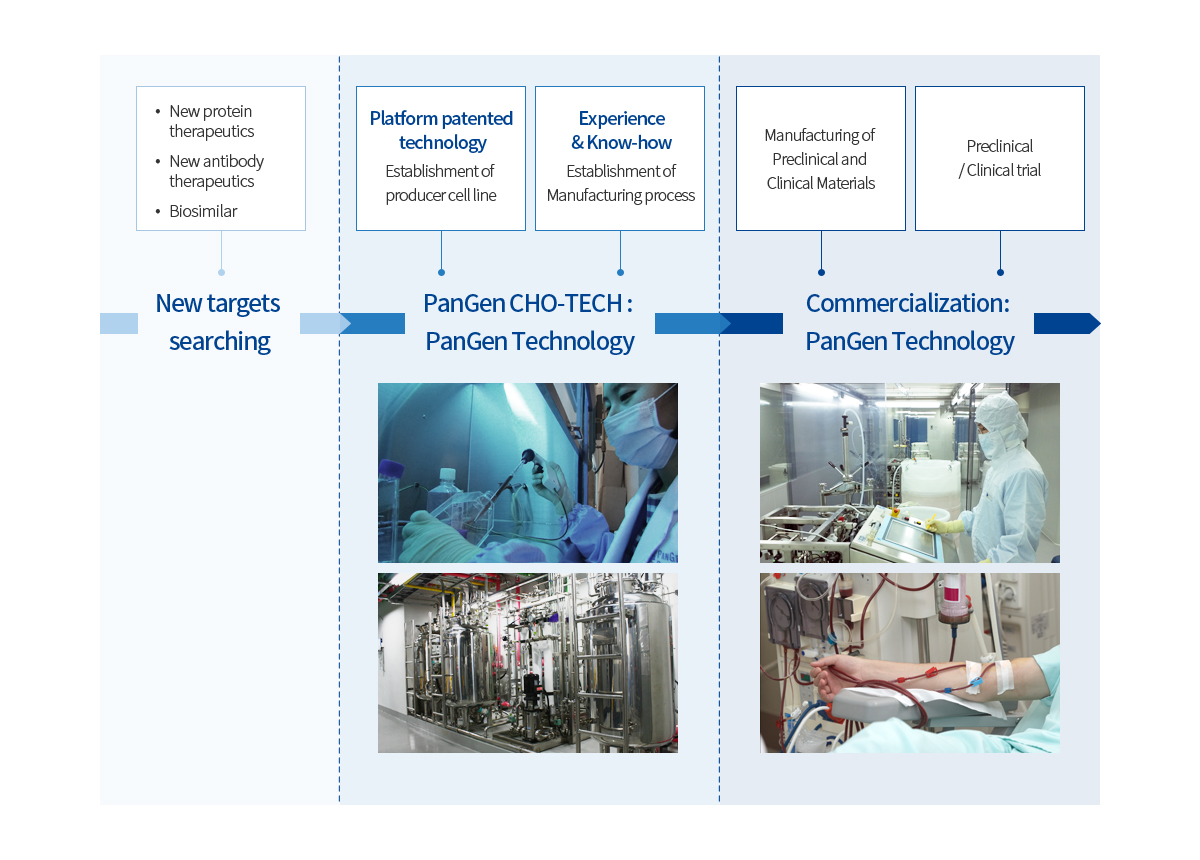
The platform patented technology on establishment of the producer cell lines is the core success factor of the biosimilars development.

Platform Technologies
PanGen is fully equipped with all the technologies required for the biologics development

PanGen CHO-TECHTM

- Importance of technology
-
- Protein expression technology for producer cell line and manufacturing process development
- The technology for developing producer cell lines is the most important factor for the successful biologics development.
- Superiority of technology
-
- Maximization of protein expression efficiency: Increased gene expression efficiency about 200 times or more compared to the existing technologies
- Maximization of expression stability: Stability of expression for longer than 90 days of continuous culture (50~60 days by existing technologies)
- Minimization of the development period: Shortened development time for producer cell line establishment to less than 5 months
GMP Manufacturing Facility Secured
- The manufacturing plant specialized for biologics with 50 ~ 1,000L capacity
- Early securing of GMP manufacturing facility before preclinical stage significantly increase the success possibility of biosimilar product development.
- Characteristics of PanGen GMP facility
-
- Designed in accordance with the European GMP Guideline
- Optimized GMP facility for manufacturing of protein therapeutics
- One-stop service available from producer cell line development to manufacturing of clinical trial materials
- Feasible manufacturing cost reduction by applying disposable bioreactors for the first time in Korea
- Status of facility and future plan
-
- Due diligence on GMP facility has been successfully approved for KFDA and PIC/S.
- Securing process reproducibility and quality consistency in commercial manufacturing scale prior to the initiation of the clinical trials
- Upon the launch of recombinant Factor Ⅷ, the facility will be mainly dedicated to the manufacturing of PanGen’s biosimilar EPO and recombinant Factor VIII
- WFI system
-

- Disposable bioreactor and purification systems
-




