Products
PRODUCTS
Technology Transfer Service
Quality Service
- 1
-
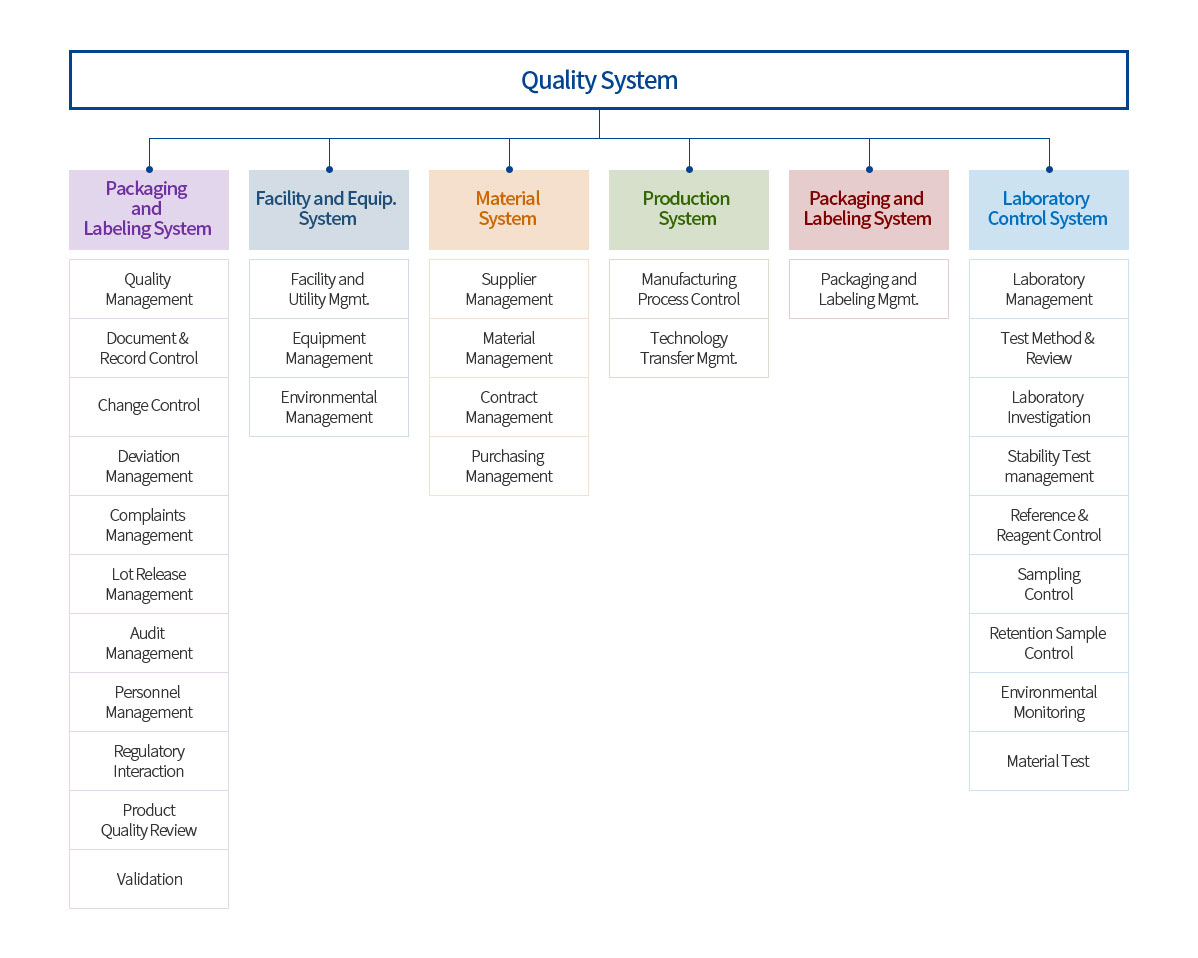
Establishment of a company-wide quality management system covering all stages from the research and development stage of pharmaceuticals to the final stage of supply to patients
- 2
-
Production management of pharmaceutical products that meet safety, stability, efficacy and GMP standards through a continuous quality management system
Quality System

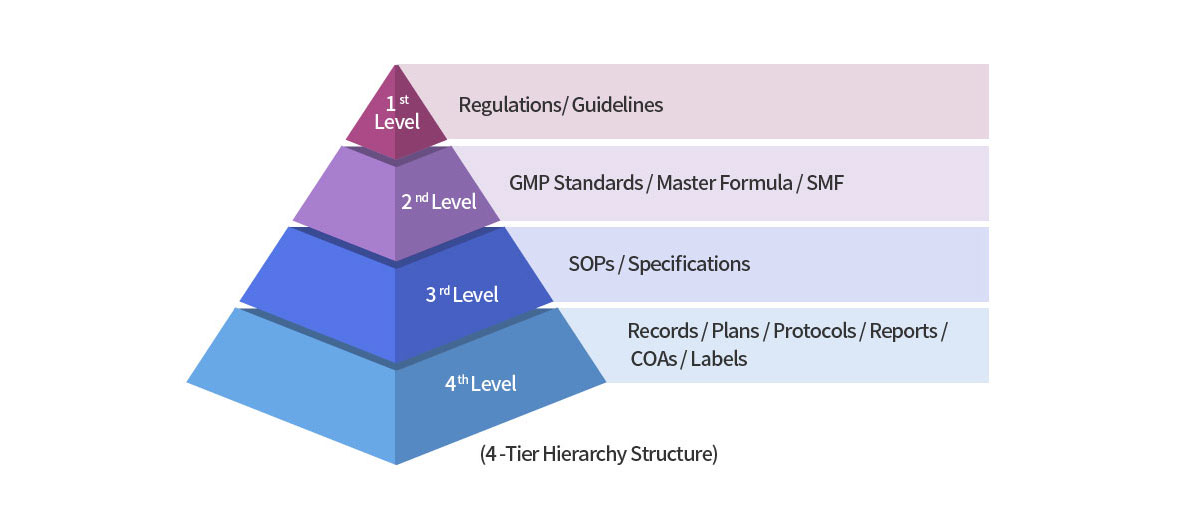
Document System

Analysis Services

-
-
Lot release test
-
Stability test
(Long-term, Accelerated, Stress, Photo stability) -
Bulk product test
-
Material test
-
In-coming test
-
Pharmaceutical water test
-
Purity test
-
Identification test
-
Content test
-
Quantification test
-



